Mycofast U.S.®
Culture-Enumeration-Identification-Susceptibility testing
MYCOFAST U.S. has been designed for the detection, identification, and enumeration of Ureaplasma urealiticum (U.u.) and Mycoplasma hominis (M.h.) in endocervical, urethral, urinary, gastric,and sperm specimens.
- Mycofast U.S.
- Instructions for Use
- Request a Quote
- Brochures and Literature
- Methodology
MYCOFAST U.S.®
Detection, identification, and enumeration
in one simple test.
MYCOFAST U.S. has been designed for the detection, identification, and enumeration of Ureaplasma urealiticum (U.u.) and Mycoplasma hominis (M.h.) in endocervical, urethral, urinary, gastric,and sperm specimens.
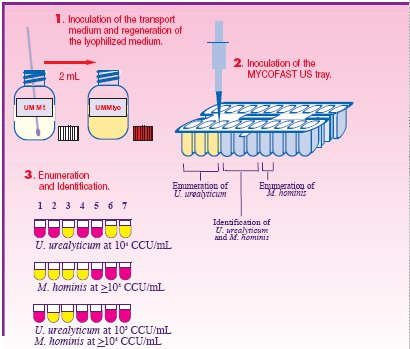
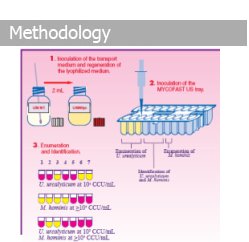
Results from the MYCOFAST U.S. test are comprehensive. Wells 1-3 enumerate for U.u. between 103 and ≥105 CCU/mL. Wells 4-6 for the identification of U.u. and M.h. via resistance profiles to Lincomycin (L), Trimethoprim /Sulfamethoxazole (SXT) and Erythromycin (E). Well 7 enumerates for M.h. (≥104 CCU/mL).
Using an "all liquid" method, MYCOFAST U.S. identifies U.u. and M.h. growth after 24-hours of incubation. During growth, U.u. and M.h. metabolize urea and arginine resulting in a color change of the medium. This color change is due to liberation of ammonia resulting in an alkaline pH of the medium.
Reading the results couldn’t be easier.
Yellow = Negative Pink = Positive
This test is easy to use and cost effective for all laboratories. Contact us now to set up a free in-lab demonstration.
Each microplate is divisible allowing for single or multiple sample testing.
MYCOFAST U.S. provides SENSITIVE, SPECIFIC, AND RAPID results in a complete kit.
Each kit includes 30 tests:
UMMt: Liquid medium for sample transport.
UMMlyo: Lyophilized growth medium for better stability. To be reconstituted in the laboratory with the inoculated UMMt.
M.h. Supplement: Growth activator for M. hominis detection within 24 hours.
MYCOFAST U.S. tray: Divisible tray (2 x 10 wells).
To view these documents you need to have Adobe Acrobat Reader.
You can get it by clicking here.
Mycofast U.S. Instructions For Use
To view these documents you need to have Adobe Acrobat Reader.
You can get it by clicking here.
FDA Listing
U.S. FDA Listing for Mycofast/Mycoscreen PlusBrochures
Mycofast U.S. BrochureArticles
Urogenital mycoplasma diagnosis, identification and sensitivity testing to 7 antibiotics----European Congress of Clinical Microbiology and Infectious Diseases